1.1 What causes keratoconus?
1.2 Classification of keratoconus
2. How to recognise keratoconus
2.1 How is keratoconus diagnosed?
2.2 Can you recover from keratoconus?
3. How is keratoconus treated?
3.1 Intrastromal ring implantation
3.2 Cross-linking treatment
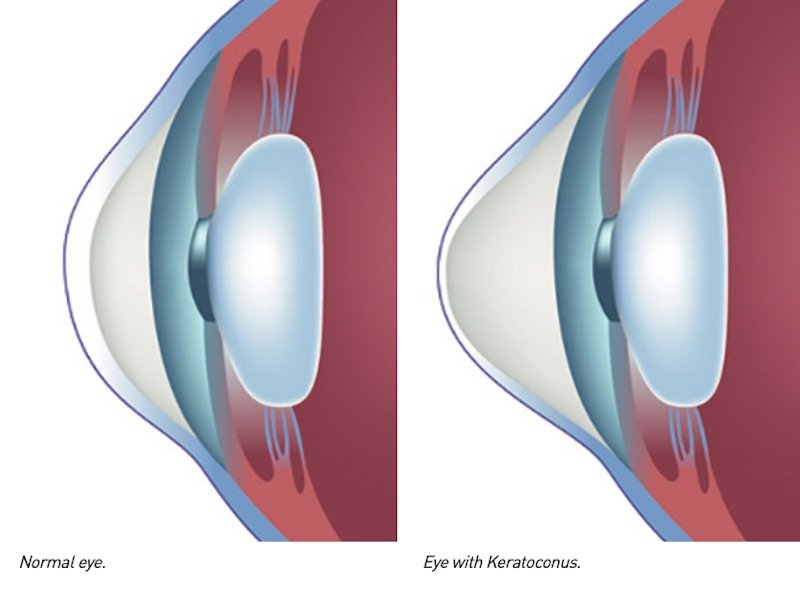
Keratoconus is a degenerative disease characterised by the progressive thinning and de- formation of the central area of the cornea, the clear membrane that forms the anterior surface of the eye.
As the disease progresses, ocular pressure causes the cornea to become distorted, the- reby losing its natural round shape by developing – initially in the posterior surface and then moving on to affect the anterior part – an irregular swelling (ectasia) in the shape of a cone.
The deformation and protrusion of the cornea affect the way light hits the retina at the back of the eye: images are focused on multiple points rather than a single point of focus, thus resulting in blurred, split and distorted vision.
Keratoconus therefore causes a refractive defect that brings with it significant problems of vision (high myopia and astigmatism), constituting serious psychological as well as physical harm to the patient.
It does not lead to blindness, but the worsening of visual quantity and quality can prevent the patient from leading a normal life.
Since the disease generally occurs during puberty, affecting young people with a long life ahead of them and the hope of maintaining their eyesight, the importance of early diagnosis and timely treatment is clear.
The cause of keratoconus is still unknown, although recent research seems to in- dicate that it may derive from a combination of genetic and environmental factors. Some cases of keratoconus have a hereditary component: in fact, it has been shown that about 20% of patients have relatives who are also affected by the disease. Other genetic diseases are also associated with keratoconus, including trisomy 21 (Down syndrome), Marfan syndrome, and Ehlers-Danlos syndrome.
Keratoconus may also develop more easily in people who suffer from systemic dise- ases or who have had previous eye problems such as allergic conjunctivitis, retinitis pigmentosa, and Leber congenital amaurosis.
Lastly, it is possible that, in those particularly predisposed, the continuous use of contact lenses and excessive eye rubbing may cause microtraumas to the cornea which “trigger” the start of the disease.
Keratoconus evolves progressively in four stages, from a mild form (the so-called forme fruste keratoconus which is often asymptomatic and confused with other eye- sight defects) to the extremely severe stage in which the thinning of the cornea is at maximum, the ectasia is visible to the naked eye and myopia and astigmatism can no longer be corrected with glasses or contact lenses.
If the traditional classification of the evolution of this disease has as its parameters the thinning and the progressive curvature of the cornea, a second and no less im- portant method of classification of keratoconus is based on morphology, i.e. on the shape the corneal deformation assumes, and on the position of ectasia in relation to the centre of the eye.
By means of a specific examination (pachymetry), the thinnest point of the cornea can be determined and the distance from the centre of the pupil to this point is measured. This measurement is used to classify keratoconus as either central, paracentral or pericentral and can therefore be categorised as:
● Nipple keratoconus: central or paracentral with high asphericity
● Bowtie keratoconus: central with regular astigmatism
● Croissant keratoconus: para-or pericentral
● Duck keratoconus: paracentral
● Snowman keratoconus: paracentral
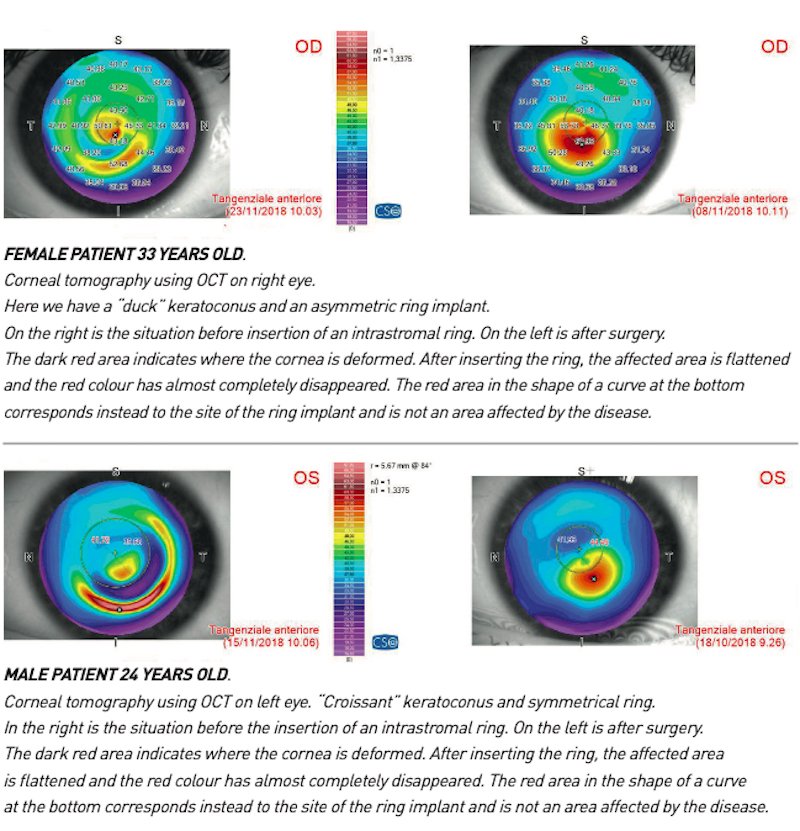
This type of classification – developed by the Spanish ophthalmologists Luis Fernán- dez-Vega and Jose Alfonso – is extremely important as it allows the surgeon to per- form implant surgery of intracorneal rings (Kerarings[1]), which, by flattening the cornea, help to improve or eliminate the refractive defect caused by the corneal deformation and contribute to slowing down the progression of keratoconus, giving back the patient the highest quality and quantity of vision possible.
The Fernández-Vega Alfonso Classification

A sense of discomfort towards light or glare (photophobia), itching and the need to rub the eyes frequently, appearance of halos around lights at night and the unpleasant feeling of seeing people and objects, both from near and afar, as blurred and distorted…
These are the initial symptoms of keratoconus, a subtle disease, as in its early stages it can be mistaken for a refractive defect such as myopia and astigmatism and corrected with glasses or contact lenses. However, the need to frequently increase the prescription of your lenses is an alarm bell which could suggest the presence of the characteristic thinning and deformation process of the cornea, typical of keratoconus.
Finding out if one has keratoconus can have a very strong psychological effect, especially on a child or teenager. It can be a real “shock” that affects the patient’s school, work, social and family life.
However, modern treatment strategies make it possible to diagnose the disease early and block its progression, as well as any consequential future damage not only to the patient’s eyesight but also to their quality of life.
Since the onset of the disease usually occurs in childhood or adolescence, early diagnosis is very important to avoid any progression of keratoconus!
All children should have an eye exam around the age of three to ensure that both eyes are properly coordinated, have good vision and are healthy in general. If there is any family history of keratoconus, we recommend a more thorough examination, possibly including corneal topography, as early as 5-6 years of age.
Diagnosis of keratoconus can be made at an eye clinic equipped with the specific equipment capable of mapping the cornea by measuring its thickness and shape.
Among these, the corneal topographer is particularly important: in fact, this instrument creates a coloured topographic map of the anterior surface of the cornea which can hi- ghlight any deformations and the development of the characteristic cone shape. Altitudinal corneal topography can also be reliably performed on schoolchildren and is therefore very useful in detecting even the earliest signs of keratoconus.
The topography is accompanied by other specific tests such as corneal pachymetry. This examination makes it possible to measure the thickness of the cornea. Different investi- gation techniques are available: Scheimpflug corneal tomography (non-contact optical pa- chymetry with Sirius or Pentacam camera) which provides a pachymetric map of the cor- nea as well as pachymetry using an ultrasound probe (acoustic pachymetry – contact type).
Corneal tomography provides a complete “photograph” of the thickness of the cornea by measuring both the anterior and posterior corneal surface.
Topography and tomography can be performed using Scheimpflug Camera technology (Si- rius – Pentacam) or by OCT (MS-39, CASIA).
Altitudinal corneal topography, pachymetry and corneal tomography are simple, fast, painless and indispensable eye tests!
In the first stage of the disease, keratoconus does not in fact generate obvious corneal de- formations or a substantial thinning of the cornea and significant corrections to the patient’s eyeglass prescription is not necessary. Only an accurate and reliable corneal topography or a corneal tomography can detect any changes in corneal shape.
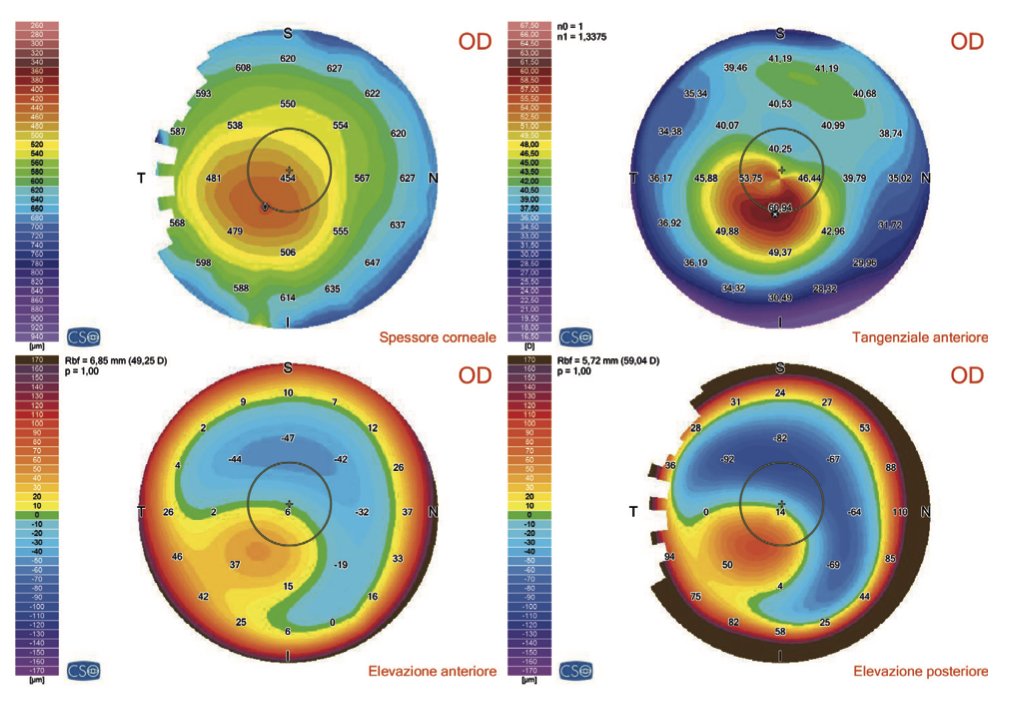
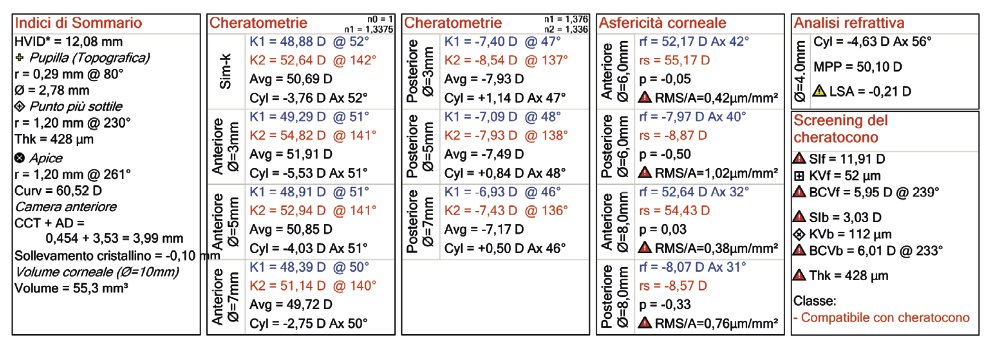
Corneal topography (or tomography) taken with OCT technology (MS-39).
The more intense central red area indicates a deformation of the cornea.
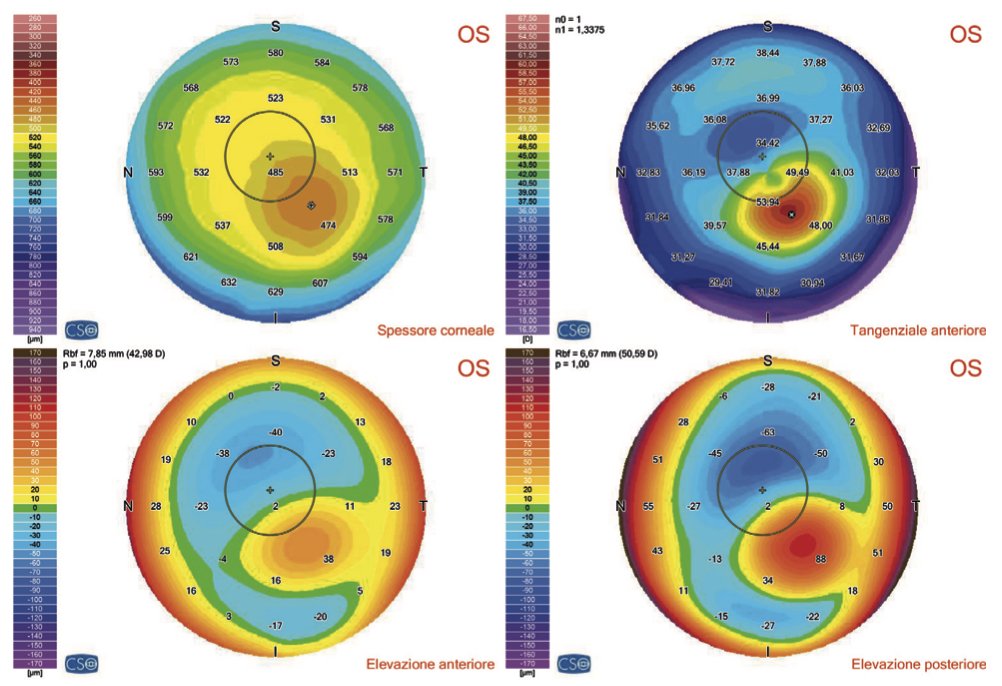
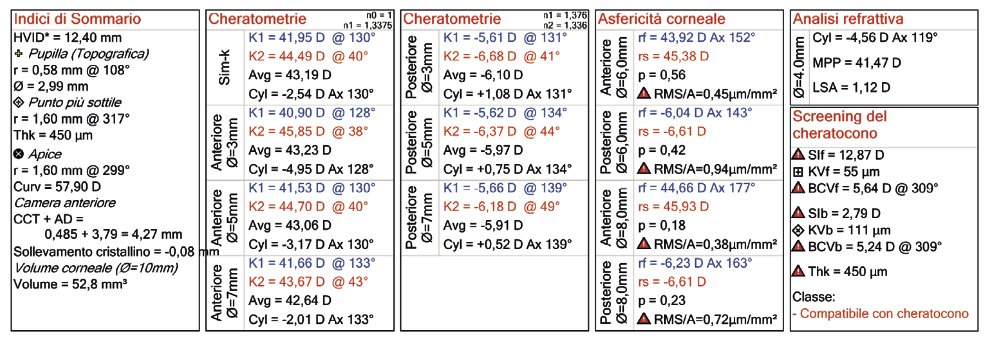
Another example of an OCT exam indicating symptoms compatible with keratoconus.
Corneal topography (or tomography) taken with OCT technology (MS-39).
There are currently no pharmacological treatments that can completely get rid of kera- toconus from the diseased eye.
However, there are very successful corneo-plastic treatments that can stop the progression of the disease and give the patient a normal social and working life. Early treatment after diagnosis is therefore essential.
Corneo-plastic surgery is the innovative combination of corneal and refractive surgery techniques that can correct numerous visual defects, preserving the natural anatomy of the cornea as much as possible. In the case of keratoconus, the most effective cor- neo-plastic treatments are:
● Corneal cross-linking to stop the degenerative processes of the disease. Cross-linking can stimulate the renewal of corneal cells (keratocytes) that then start producing healthy collagen again. This causes an increase in corneal thickness and the reduction of corneal ectasia.
● Implantation of intrastromal rings (Kerarings[1]) to regress the corneal decay, by “flattening” the cornea and bringing it back to its natural shape.
This can be combined with the use of special contact lenses or the implantation of pha- kic intraocular lenses (PIOL) to correct refractive defects.
Is one method better than the other? Certainly not!
Each treatment strategy is highly personalised to the patient’s eye. Depending on the degree of development and the type of keratoconus diagnosed, the surgeon will choose and discuss the best therapy together with the patient. Here is an outline of possible solutions (see next page).
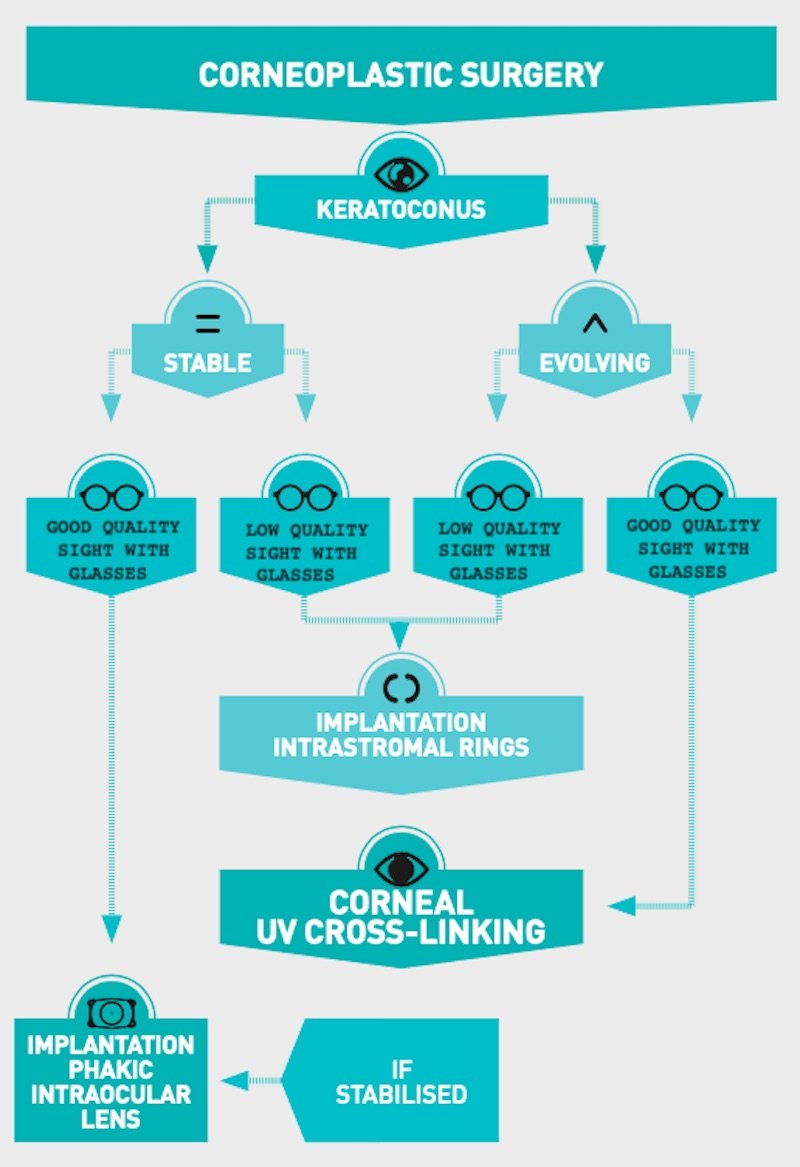
Stop the progression of the disease, allow the patient to have the best sight possible without resorting to cornea transplant surgery. This is the aim of modern keratoconus treatments.
As mentioned above, keratoconus is not treated with drugs but through treatments used together which, when used appropriately, can lead to the best possible results in the context of each individual patient.
Depending on the severity and progression of the disease, there are different ways to manage keratoconus:
● in the initial stages, the decrease and distortion of vision can be corrected with glasses but as the disease progresses, the cornea becomes highly irregular and it is then necessary to wear specific rigid contact lenses in order to adequately correct the patient’s sight;
● rigid contact lenses may be the only way to see well in cases where the cornea has become totally deformed by keratoconus (in these cases no ‘non-contact’ correction is able to sufficiently correct the patient’s sight defect). Contact lenses for keratoconus are like an artificial cornea with a spherical outer part and inner part that, by way of the tear film, adhere to the irregular anterior corneal surface;
● if contact lenses also become inadequate or are intolerable, it is possible to fit intrastromal ring implants (Kerarings[1]) in order to flatten and make the shape of the distorted cornea regular again and thus improve vision;
● implantation of intrastromal rings is most effective when used in conjunction with corneal cross-linking (CXL) so as to achieve further corneal stabilisation. Cross-linking (standard or transepithelial with iontophoresis) is an innovative chemical-physical treatment that, through the combined use of riboflavin (vitamin B) and ultraviolet light, helps to strengthen the cornea, therefore halting the progression of keratoconus;
● corneal grafts (corneal transplants) may be necessary for the most severe and advanced cases of keratoconus. However, corneal transplantation is invasive and not without risks; it is therefore not the main goal, but a last resort that is best if we can avoid it!
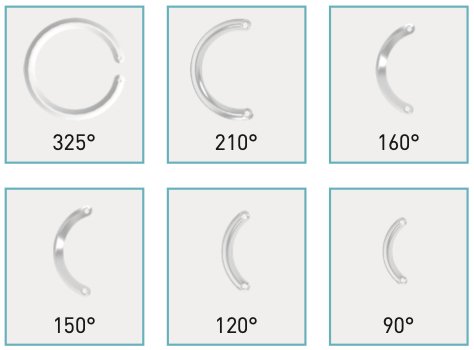
Stop the progression of the disease, allow the patient to have the best sight possible wi- thout resorting to cornea transplant surgery. This is the aim of modern keratoconus tre- atments.
Intrastromal rings are a minimally invasive surgical option designed to stabilise or de- lay the evolution of keratoconus.
Their function is to correct the deformation of the cornea, reduce refractive defects (in particular high myopia and irregular astigmatism) and therefore improve visual acuity.
If the ring implant does not completely free the patient from having to use contact len- ses or glasses, it greatly improves quality of life: tolerability to contact lenses is much greater and, in some cases, it is possible to abandon them altogether and use regular glasses.
What are intrastromal rings?
Kerarings are transparent plastic rings made up of two semi-circular segments of variable diameter, curvature and thickness. After careful analysis of the characteristics of the patient’s keratoconus and based on the morphological classification of doctors Fernández-Vega and Alfonso, the surgeon then chooses the most suitable type of rings.
The rings are made of artificial material, Perspex CQ Acrylic, which is the same material used for more than 20 years in refractive surgery for the implantation of artificial intraocular lenses or for the replacement of the opaque crystalline lens due to cataracts. It is therefore a tried and tested material that does not present risks of rejection.
The rings are inserted surgically by using a Femto Laser which is able to create a tunnel of the desired size and with the appropriate characteristics.
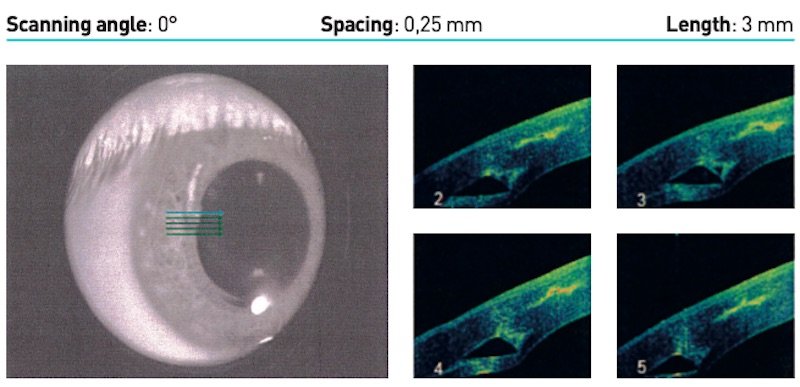
In this corneal tomography using OCT, the right-hand tunnel made by the operator by hand (without Femto Laser) can be clearly observed and is in too superficial a position. The implanted ring, showing visible extrusion, had to be removed.
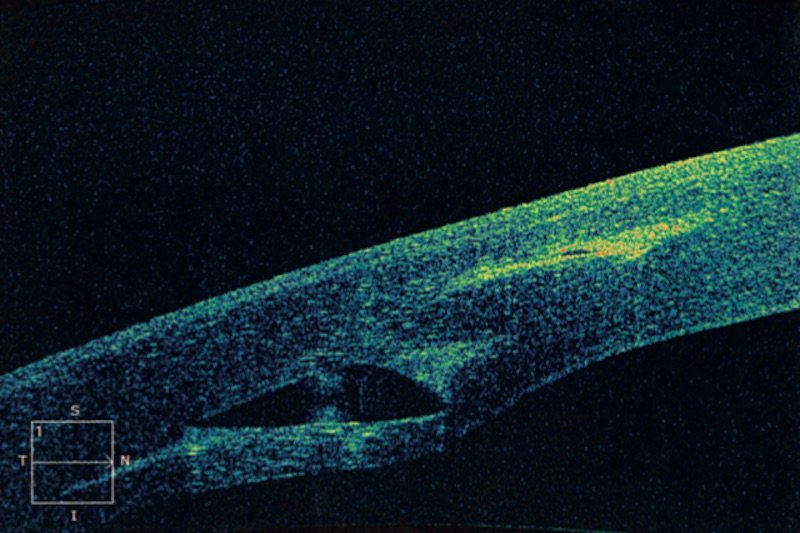
On the left, you can see the 6mm diameter keraring ring correctly positioned at about 80% of the depth of the corneal thickness in the Femto Laser tunnel. This image is very interesting as it demonstrates the absolute superiority of the Femto Laser in the surgery of intrastromal rings.
How the procedure is carried out
The surgical procedure is performed on an outpatient basis while the patient is awake. The rings are in fact implanted under topical anesthesia, i.e. by instilling a few drops of anesthetic eye drops into the eye.
Using a high-precision Femtosecond Laser, the surgeon creates a “pocket” in the area of greatest corneal thickness (the stroma) and inserts the rings. The entire procedure only takes about 15 minutes.
Visual rehabilitation is almost immediate and post-operative pain almost non-existent.
If necessary, a protective contact lens is applied after surgery which is then usually removed the day after the operation. The use of antibiotic and anti-inflammatory eye drops allow for a more comfortable and secure post-operative period and, generally after 3 days, the patient can return to their usual routine.
The most advanced approach to keratoconus therapy is based on the synergy between the latest therapeutic solutions available: since the disease progresses constantly and unpredictably, after the Keraring implant, it is thus highly advisable to perform a risk- free treatment such as cross-linking with iontophoresis to further stabilise the cornea.
In fact, the two techniques reinforce one another and give exceptional results!
Waiting is not the right strategy!
A patient, a 40-year-old man, came for a check-up for the first time in 2017 with keratoco- nus. The therapeutic indication was the implantation of an intrastromal ring.
At that time, the patient had a minimum tunnel depth of 518 microns for implanting the ring.
As shown by the simulation, shown in image A, at that time it was possible to implant a ring with a thickness of 300 microns, which could be accommodated in the tunnel with a minimum depth of 518 microns.
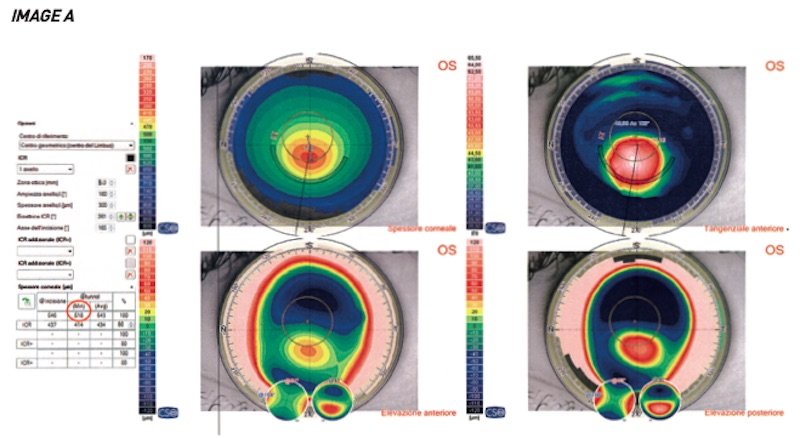
The implant area of the cornea that will host the ring is studied in advance through corneal topography. A virtual construction of the tunnel is carried out at a certain depth. At this point, the operator decides whether the minimum thickness of the virtual tunnel (which will be positioned at 80% of the corneal thickness from the external surface) is sufficient to accommodate a given ring thickness based on the nomogram.
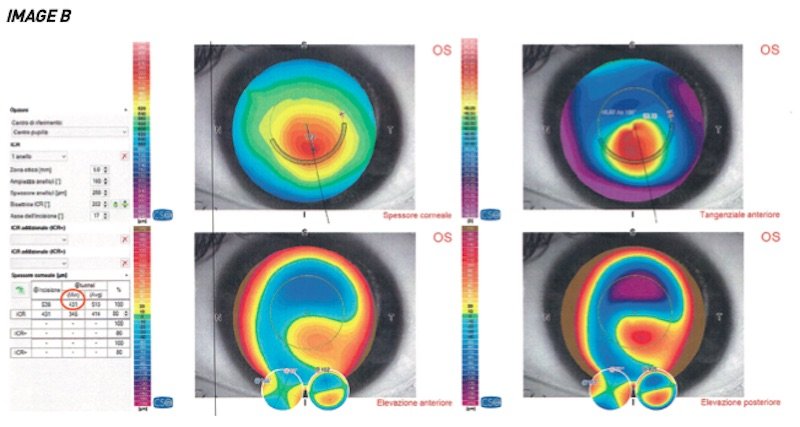
However, the patient decided not to undergo surgery and returns for another check-up at the end of 2018.
A new exam is carried out, as shown in image B, the result of which shows a significant loss of corneal thickness. In fact, in less than 2 years we have passed from an available tunnel with a minimum height of 518 microns to 431 microns. A loss of 87 microns.
It is still possible to implant a ring in this case, but it will have to be at a height of 250 microns and will no longer be able to completely correct the visual defect.
Waiting and putting it off can be very risky, as if the progression of the disease continues to be so rapid, you can definitely miss the opportunity to block the wearing out of the cornea with intrastromal rings.
To date, transepithelial cross-linking with iontophoresis is definitely one of the best weapons at our disposal to block keratoconus.
What does it involve?
Cross-linking is a parasurgical treatment aimed at stopping the degenerative pro- cess of keratoconus. The principle on which it is based is to stimulate the formation of cross-links between the collagen fibres that make up the load-bearing structure of the cornea.
Keratoconus is caused by the weakening and alteration of the chemical bonds between the fibres: the cornea loses its capacity for mechanical resistance and tends to deform progressively under the action of eye pressure.
Through cross-linking, the cornea is strengthened, making it stiffer and thus stop- ping its wearing out.
There are two types of cross-linking
● standard corneal cross-linking
● transepithelial cross-linking
Standard corneal cross-linking is epi-off. This means that the epithelium is removed and must therefore be done in a sterile room, as it presents risks. This procedure takes less than one hour. It involves making a photosensitive substance, riboflavin (vitamin B2), react with ultraviolet light, generated by a specific device.
The treatment is carried out in several stages:
1. first the patient receives eye drops to narrow the pupil, then a second set of anesthetic eye drops is applied;
2. the surgeon gently removes the epithelium, the thin layer of cells that cover the cornea, and soaks it with riboflavin, repeating the operation every 3 minutes for about a quarter of an hour;
3. once the cornea has absorbed the correct amount of riboflavin, it undergoes low intensity ultraviolet irradiation for about 30 minutes, which is in absolutely no way harmful to the eye;
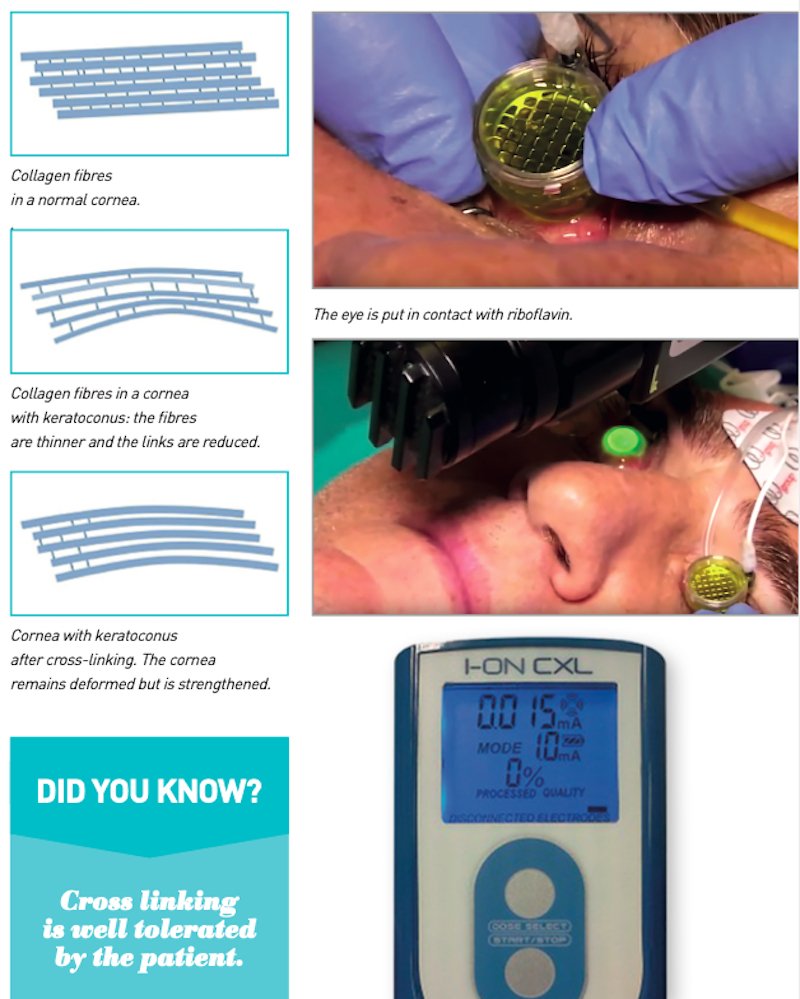
4. the combination of riboflavin and ultraviolet light (both natural elements found in nature) creates a chain of reactions within the cornea that lead to the formation of new healthy corneal collagen that is not degenerated like that of a cornea affected by keratoconus;
5. at the end of the procedure, the treated cornea is covered with a protective contact lens and left in place for 3-5 days until complete re-epithelialisation.
Even less invasive, and with greater tolerance among patients, is transepithelial cross-linking which, as the name suggests, allows riboflavin to pass through the epithelium without having to remove it. This makes the procedure free from possible infectious risks and post-operative pain.
In this innovative technique, riboflavin penetrates the eye tissue by means of ionto- phoresis, a technique of transporting the drug across the eye structures by way of a low-voltage electrical current.
Here is how iontophoresis works
The treatment is carried out by applying two electrodes to the patient: the negative electrode is applied by suction, using a specific ring, to the cornea to be treated; the positive electrode is positioned on the patient’s forehead using a small plaster-like “patch”.
By using the physical principle of ionic migration from one electrode to the other, specific polarised drugs can be prepared that contain positive or negative ions, or both (bipolar) and applied to the electrodes according to their polarity.
Just like the wagons of a train carry goods by following the rails, the low voltage current, used for about 5 minutes, carries the polarised drug across the tissues, as the ions of the drug itself migrate to the opposite electrode until the drug is completely absorbed.
By means of iontophoresis, riboflavin is absorbed much quicker and more effectively and treatment times are reduced considerably, to just 5 minutes.
In conclusion…
● Keratoconus is a serious disease that should not be underestimated.
● For many young patients, it can turn into a life sentence.
● Early diagnosis plays a fundamental role in the treatment strategy
● There are valid and proven treatments to stop the evolution of the disease and restore the highest possible quality of vision to patients.

… Let’s not let an initially small problem turn into a big problem
Biography of Dr. Alberto Bellone
He graduated in Medicine and Surgery at the University of Turin in 1996 and specialized at the same University in Ophthalmology in 2000.
He is among the best and most successful Italian refractive surgeons and deals in particular with:
● Cataract surgery with high-tech IOL implantation
– Performed the first implantation of Panoptix trifocal lenses
– Also acquired extensive experience in the management of multifocal and toric IOLs
– Implanted hundreds of multifocal IOL lenses
● Conservative keratoconus surgery through corneal cross-linking and insertion of intrastromal corneal rings
● Surgery with Excimer Laser
● IOL phakic implant: he is a certified surgeon by the US company Staar
● In June 2016, he implanted the first ICL Evo + v5 lens in Italy
Established vitreoretinal surgeon, he routinely performs minimally invasive vitrectomies and has performed over 350 27 gauge vitrectomies to date.

For those wishing, we point out the most recent studies on the subject.
KERARING
Four-Stage Procedure for Keratoconus: ICRS Implantation, Corneal Cross-linking, Toric Phakic Intraocular
Lens Implantation, and Topography-Guided Photorefractive Keratectomy.
Coskunseven E, Sharma DP, Grentzelos MA, Sahin O, Kymionis GD, Pallikaris I.
J Refract Surg. 2017 Oct 1; 33 (10): 683-689. doi: 10.3928 / 1081597X-20170807-01. PMID: 28991336
Femtosecond laser implantation of a 340-degree intrastromal corneal ring segment in keratoconus: Short-term outcomes.
Sadoughi MM, Einollahi B, Veisi AR, Zare M, Sedaghat MR, Roshandel D, Einollahi N, Rezaei J.
J Cataract Refract Surg. 2017 Oct; 43 (10): 1251-1256. doi: 10.1016 / j.jcrs.2017.07.026. PMID: 29120710
Femtosecond-assisted intracorneal ring segment complications in keratoconus: from novelty to expertise.
Mounir A, Radwan G, Farouk MM, Mostafa EM.
Clin Ophthalmol. 2018 May 22; 12: 957-964. doi: 10.2147 / OPTH.S166538. eCollection 2018. PMID: 29872254
CROSS LINKING and IONTOPHORESIS
Trans-epithelial corneal collagen cross-linking with iontophoresis for progressive keratoconus.
Ameerh MAA, Bdour MDA, Al-Till M, Faouri MA.
Int Ophthalmol. 2018 Apr 19. doi: 10.1007 / s10792-018-0920-4. [Epub ahead of print] PMID: 29675563
Efficacy of iontophoresis-assisted epithelium-on corneal cross-linking for keratoconus.
Jia HZ, Peng XJ.
Int J Ophthalmol. 2018 Apr 18; 11 (4): 687-694. doi: 10.18240 / ijo.2018.04.25.
eCollection 2018. Review. PMID: 29675392
INTRAOCULAR LENSES
Safety and Visual Outcome of Visian Toric ICL Implantation after Corneal Collagen Cross-Linking in Keratoconus: Up to 2 Years of Follow-Up.
Antonios R, Dirani A, Fadlallah A, Chelala E, Hamade A, Cherfane C, Jarade E.
J Ophthalmol. 2015; 2015: 514834. doi: 10.1155 / 2015/514834. Epub 2015 Mar 19. PMID: 25874116
Three-year follow-up of posterior chamber toric phakic intraocular lens implantation for the correction of high myopic astigmatism in eyes with keratoconus.
Kamiya K, Shimizu K, Kobashi H, Igarashi A, Komatsu M, Nakamura A, Kojima T, Nakamura T.
Br J Ophthalmol. 2015 Feb; 99 (2): 177-83. doi: 10.1136 / bjophthalmol-2014-305612. Epub 2014 Aug 21.